Therapeutic Use Exemption (TUE) Committee Coverage on Glucocorticoid (GC) Injections for Hay Fever
Methylprednisolone (Depo-Medrone) and triamcinolone (Kenalog†) administered by intramuscular (IM) injection as therapy for hay fever are prohibited in-competition in sport and subsequently their use requires the athlete and their doctor to strictly adhere to the TUE Coverage. The TUE Coverage is offered at www.sportireland.ie/anti-doping/athlete-zone/athlete-zone/therapeutic-use exemptions.
Requests for IM glucocorticoid injection TUEs used to deal with hay fever are extraordinarily unusual and are hardly ever authorised. Any request ought to embody complete proof of ongoing specialist session and clear proof of the failure of non-IM glucocorticoid approaches.
- Athletes included of their Worldwide Federation Registered Testing Pool and/or athletes competing at Worldwide Competitors who require this injection through the WADA GC Washout Interval or the in-competition interval ought to contact their sport’s Worldwide Federation for recommendation on their particular TUE coverage/apply previous to administration of the therapy. Their sport’s Anti-Doping Officer can present help in figuring out Worldwide Competitions and establishing the necessities for a TUE Software (See WADA GC Washout Interval desk beneath).
- Athletes included within the Sport Eire Registered Testing Pool (who usually are not competing at an Worldwide Competitors; see above) ought to apply to Sport Eire for a Pre-test Therapeutic Use Exemption upfront of receiving an IM glucocorticoid, if the injection administration will happen through the WADA GC Minimal Washout Interval or the in-competition interval. Their TUE utility must be ready utilizing the knowledge within the Medical File part beneath to assist them (See WADA GC Minimal Washout Interval desk beneath). The GC Injection shouldn’t be administered till Sport Eire’s TUE Committee choice has been communicated to the athlete.
- Athletes eligible for a Put up-test TUE utility (see www.sportireland.ie/anti-doping/athlete-zone/athlete-zone/therapeutic-use-exemptions) ought to be sure that previous to the administration of any IM glucocorticoid injection by a doctor, they’re able to getting ready a medical file to the usual outlined beneath, if the injection administration will happen through the WADA GC Minimal Washout Interval or the in-competition interval. Athletes could also be required to submit this medical file to help a TUE utility later. The TUE utility must also be ready utilizing the knowledge within the Medical File part beneath (See WADA GC Minimal Washout Interval desk beneath).
† Be aware: Whereas Kenalog (triamcinolone acetonide) injection has been discontinued from the Irish market some unlicensed product could also be obtainable.
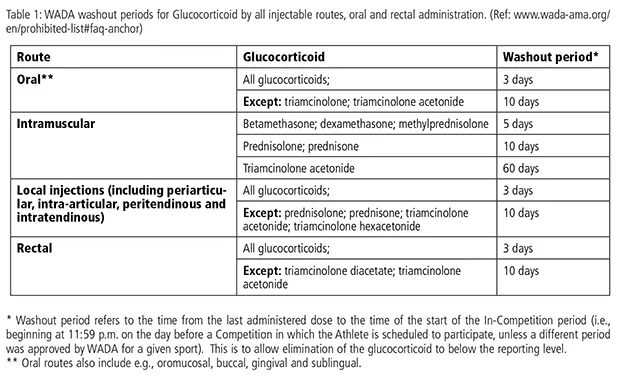
WADA Washout Intervals
Glucocorticoids are prohibited in-competition solely when administered by injectable, oral or rectal routes. WADA have launched Washout intervals for GC use by all routes of injection, orally and rectally of which all physicians treating athletes topic to doping management must be conscious (see Desk 1). Out-of-competition use of GC may lead to an in-competition Opposed Analytical Discovering (AAF); subsequently, you will need to take into account the WADA washout intervals when figuring out an athlete’s therapy. When it comes to hay fever therapy, IM injection of triamcinolone acetonide now has a 60-day washout interval whereas IM injection of methylprednisolone has a 5-day washout interval.
Medical File
IM glucocorticoid TUE purposes MUST be accompanied by a medical file reflecting present greatest medical apply to incorporate:
- A whole medical historical past i.e., when the hay fever started; the related signs, their severity and impact on sporting efficiency; and signs suffered in earlier hay fever episodes.
- Medical proof of making an attempt to make use of various permitted oral, nasal and/or ophthalmic medicines and justification as to why various permitted medicines usually are not ample.
- Copies of all related examinations, laboratory outcomes/studies and scientific notes (for instance, if a clinic go to is referenced in a letter or abstract, a duplicate of the scientific notes taken through the go to should be submitted); present particulars of any identified allergens or allergic historical past together with outcomes of any earlier immunological testing.
- Actual identify, speciality, handle (together with phone, e-mail, fax) of analyzing doctor.
Prescribers are reminded that assets can be found to help in figuring out whether or not a drugs is prohibited or permitted in sport. See www.sportireland.ie/anti-doping/athlete-zone/athlete-zone/how-to-check-your-medications
Permitted Drugs
Athletes and their physicians are reminded that there are a number of permitted medicines, each over-the-counter and prescribed, thatcan be used for the therapy of hay fever (standing as checked on the MedCheck Database on twenty first March 2025) resembling:
Over-the-counter medicines (examples)
- Oral Antihistamines: e.g., loratadine (manufacturers – Clarityn, Lorat and so on.), cetirizine (manufacturers – Cetriz, Cetrine Allergy), chlorphenamine(model – Piriton), fexofenadine hydrochloride (model – Telfast Allergy)
- Decongestant Sprays: e.g., Otrivine Sinusitis Reduction Nasal Spray, Otrivine Congestion Reduction Menthol 0.1% w/v Nasal Spray
- Glucocorticoid Nasal Sprays: e.g., Beconase Hayfever, Flixonase Allergy Reduction
- Eye Drops: e.g., Otrivine-Antistin eye drops, Opticrom Allergy eye drops
Prescribed medicines (examples)
- Oral Antihistamines: e.g., Neoclarityn, Telfast
- Oral Allergen Extracts: e.g., Grazax, Oralair
- Glucocorticoid Nasal Sprays: e.g., Avamys nasal spray, Rhinolast nasal spray, Nasonex nasal spray
Up to date April 9, 2025
